We measure experience in decades.
With an average of more than 20 years of industry experience per team member, you’ll know your project will be handled by only senior-level professionals.
Since our inception in 2000, our exceptionally low turnover rate has ensured that EMB clients benefit from the reliability and consistency of collaborating with the same expert associates on subsequent projects. In this industry, stability and reliability are crucial.

Meet the EMB Team

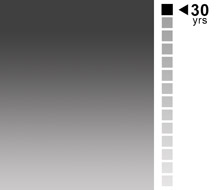
Ed Brown, Ph.D.
Executive Director
From managing medical research data to teaching biostatistics to supporting theoretical statistics via numerical analysis, Ed’s career has spanned an extraordinary range. He left the academic arena to start EMB with a close group of colleagues from the university and Pharma. With more than 30 years of industry experience, he’s compiled a wealth of knowledge that enables EMB to continue its steady growth under his leadership.
EMB Biostatisticians
Seasoned leaders of numerous complex studies, EMB offers biostatistics expertise found in few other CROs.

Mike Mosier, Ph.D.
Director, Biostatistics
Natural lead with diverse industry experience and lots of FDA face time. Well-published, Mike remains academically active and enhances our projects with his marathoner’s drive and fortitude.
Thangam Arumugham, Ph.D.
Principal Biostatistician
Comprehensive industry experience in early trial studies across numerous therapeutic areas. Thangam’s depth of knowledge, technical skills and meticulous nature makes her an invaluable team member.
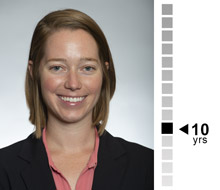
Kelsey Brown, M.S.
Senior Biostatistician
Solid experience in Phase I and II studies with diverse designs and spanning numerous indications. Kelsey’s organized and disciplined attention to detail make her a naturally strong lead.

Dave Donley, Ph.D.
Principal Biostatistician
Specialist in the design, analysis and publication of Phase II-IV clinical trials, as well as serving on DSMBs. Dave has experience in a wide range of indications with expertise in adaptive design.

Dawn DuBois, M.S.
Principal Biostatistician
Meticulous and detail-driven lead, thereby a frequently requested and respected member of any team. Dawn’s wealth of experience includes FDA Advisory Panel presentation and ten NDAs.

Karla McKenzie, M.S.
Senior Biostatistician
Provides critical behind-the-scenes team support with her extensive knowledge in the analysis and reporting of studies. Karla’s industry work is impressive, making her an invaluable team member.

Brian Mosier, Ph.D.
Biostatistician
Published work in studying biomarkers under the Euclidean distance in trichotomous and 3D settings, Brian provides EMB with the latest approaches to data science.

Bruce Morrill, M.S.
Principal Biostatistician
Impeccable credentials in Phase I-III studies with 18 NDAs. Although Bruce continues to work on randomization and PK summaries, his role as EMB’s internal advisor allows him to focus on his research.
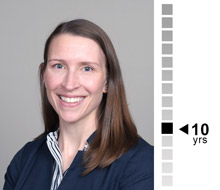
Reilly Reis, M.S.
Senior Biostatistician
Organized and diligent, Reilly is an exceptional lead. With a wide range of experience in Phase I studies, she brings specialized expertise in the design and analysis of PK and human abuse potential studies.
EMB Programmers
With extensive experience in many different therapeutic areas, our programmers have successfully directed an exceptional number of intricate and diverse studies and deliver SAS® expertise to projects large and small.

Elizabeth Dennis
Principal Statistical Programmer
Embodies the perfect combination of knowledge, precision and speed. Elizabeth’s positive leadership on projects is felt immediately, while her exacting approach and expertise with CDISC ensures success.

Ruth Johnson
Principal Statistical Programmer
A highly skilled SAS programmer on Phase I-IV studies spanning a signficant array of therapeutic areas. EMB depends on Ruth’s expertise in SDTM, ADaM, annotated CDISC CRFs, and TLF specifications.

Suzanne Maninger, MPH
Director of Programming
True cornerstone, her project leadership ranges from data management through report generation. Among her many specialties, Suzanne excels in EDC support, CDISC, and ISE/ISS data integration.

Marc Meyer, M.S.
Principal Statistical Programmer
Statistician who prefers statistical programming, which provides big-picture insight to the analyses and reports being generated. Among his many areas of expertise, Marc is our SAS/Graph specialist.
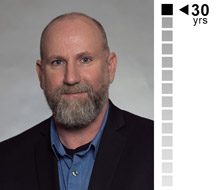
Rick Moore
Principal Database Programmer
Focused on leading EDC operations at EMB. Rick has comprehensive industry experience, including data management programming and processes, SDTM and statistical programming support.

Belinda Pierson, M.A.
Principal Statistical Programmer
Solid mathematical background and mind for analysis is the basis for an accomplished statistical programmer. Belinda is quick, accurate and detail-oriented, and she strengthens our CDISC capabilities.

Jennifer Savage Sales, M.S.
Principal Statistical Programmer
Educated in information systems while offering an in-depth programming perspective. With both CRO and Pharma experience, Jennifer has served many roles and responsibilities while providing solutions to unique problems.

Maddy Wilks
Senior Statistical Programmer
Thorough industry experience in all clinical phases, as well as comprehensive CDISC knowledge. Maddy is a solid and talented team member for any type of project.
Amy Winters
Principal Statistical Programmer
Meticulous, dedicated and precise are qualities making her a crucial team member. A key part of our CDISC group, Amy is noted for going the extra mile to ensure timelines are met for our sponsors.
EMB Data Management/Project Management
Specialists in data collection and organizing data for analysis and reporting. They embrace challenging projects, expertly manage complex studies and adapt databases to evolving needs, drawing on years of experience.

Bev Brown
Director, Data Management
Strong leader with excellent SAS skills and a broad perspective – ranging from database formation to TLG generation, Phase I to NDAs. Bev specializes in dictionary coding and laboratory data consolidation.

Melanie Edwards, MMIS, RHIA
Principal Project Manager
Biometrics project manager excelling in establishing relationships for successful delivery of studies from start up to maintenance to closeout. Focused on meeting milestones, process definition, problem solving, analytics, and risk management in compliance with regulations.

Tracie Evans, CCDM
Principal Data Manager
Detail-minded problem solver, with a proven track record in U.S. and global projects. Working with EDC systems, Tracie has established herself as a critical component to project team success.

Kim Krouse
Principal Data Manager
Top-shelf data management experience managing, reviewing, auditing research data. With a background in large, global pharmaceutical companies, Kim is a key team member on EMB data projects.

Kristin Pearson, CCDM
Principal Data Manager
Dedicated and focused data manager with effective communication skills and global experience. Kristin is specialized in CRF design using CDASH, providing EDC oversight and auditing project databases.

Lisa Spielman
Director, Project Management
A successful biometrics project manager with a history of delivering study milestones from eCRF design based on protocol requirements to management and resolution of data issues from conduct through closeout. Focused on complex problem solving, process definition, analytics, and risk management.
EMB Support Team
Critical to EMB’s success, our support professionals allow us to be at our industry’s leading edge in areas including data quality assurance, data security, business operations and information management.

Brenda Bishop, MBA
Director, Business Development

Cora Jenkins
Senior Data Quality Assurance

Lori McConnell
Director, Data Quality Assurance

Sandy Moon, MBA
Business Manager

Jeremy Pennington, CISSP
Director, Information Technology
